Explanation
Keyword for the data block. No other data are input on the keyword line.
phase name --Alphanumeric name of phase, no spaces are allowed.
Dissolution reaction for phase to aqueous species. Any aqueous species, including e - , may be used in the dissolution reaction. The chemical formula for the defined phase must be the first chemical formula on the left-hand side of the equation. The dissolution reaction must precede any identifiers related to the phase. The stoichiometric coefficient for the phase in the chemical reaction must be 1.0.
log_k--Identifier for log K at 25 o C. Optionally, -log_k, logk, -l[ og_k], or -l[ ogk].
log K --Log K at 25 o C for the reaction. Default is 0.0.
Line 4: delta_h enthalpy, [ units ]
delta_h--Identifier for enthalpy of reaction at 25 o C. Optionally, -delta_h, deltah, -d[ elta_h], or -d[ eltah].
enthalpy --Enthalpy of reaction at 25 o C for the reaction. Default is 0.0.
units --Units may be calories, kilocalories, joules, or kilojoules per mole. Only the energy unit is needed (per mole is implied) and abbreviations of these units are acceptable. Explicit definition of units for all enthalpy values is recommended. The enthalpy of reaction is used in the van't Hoff equation to determine the temperature dependence of the equilibrium constant. Internally, all enthalpy calculations are performed in the units of kilojoules per mole. Default units are kilojoules per mole.
Line 5: -analytical_expression A 1 , A 2 , A 3 , A 4 , A 5
-analytical_expression--Identifier for coefficients for an analytical expression for the temperature dependence of log K . If defined, the analytical expression takes precedence over the van't Hoff equation to determine the temperature dependence of the equilibrium constant. Optionally, analytical_expression, a_e, ae, -a[ nalytical_expression], -a[ _e], -a[ e].
A
1
, A
2
, A
3
, A
4
, A
5
--Five values defining log
K
as a function of temperature in the expression 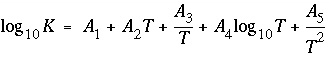 , where
T
is in Kelvin.
, where
T
is in Kelvin.