This example determines the solubility of the most stable phase, gypsum or anhydrite, over a range of temperatures. The input data set is given in table 13. Only the pH and temperature are used to define the pure water solution. Default units are millimolal, but no concentrations are specified. By default, pe is 4.0, the default redox calculation uses pe, and the density is 1.0 (not needed because no concentrations are "per liter"). All phases that are allowed to react to a specified saturation index during the batch-reaction calculation are listed in EQUILIBRIUM_PHASES, whether they are initially present or not. The input data include the name of the phase (previously defined through PHASES input in the database or input file), the specified saturation index, and the amount of the phase present, in moles. If a phase is not present initially, it is given 0.0 mol in the pure-phase assemblage. In this example, gypsum and anhydrite are allowed to react to equilibrium (saturation index equal to 0.0), and the initial phase assemblage has 1 mol of each mineral. Each mineral will react either to equilibrium or until it is exhausted in the assemblage. In most cases, 1 mol of a single phase is sufficient to reach equilibrium.
Table 13. --Input data set for example 2
TITLE Example 2.--Temperature dependence of solubility
of gypsum and anhydrite
SOLUTION 1 Pure water
pH 7.0
temp 25.0
EQUILIBRIUM_PHASES 1
Gypsum 0.0 1.0
Anhydrite 0.0 1.0
REACTION_TEMPERATURE 1
25.0 75.0 in 51 steps
SELECTED_OUTPUT
-file ex2.sel
-si anhydrite gypsum
END
A set of 51 temperatures is specified in the REACTION_TEMPERATURE data block. The input data specify that for every degree of temperature, beginning at 25 o C and ending at 75 o C, the phases defined by EQUILIBRIUM_PHASES (gypsum and anhydrite) will react to equilibrium, if possible, or until both phases are completely dissolved. Finally, SELECTED_OUTPUT is used to write the saturation indices for gypsum and anhydrite to the file ex2.sel after each calculation. This file was then used to generate figure 5.

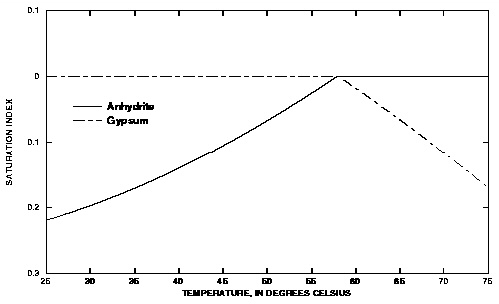
Figure 5. --Saturation indices of gypsum and anhydrite in solutions that have equilibrated with the more stable of the two phases over the temperature range 25 to 75 o Celsius.
The results of the initial solution calculation and the first batch-reaction step are shown in table 14. The distribution of species for pure water is shown under the heading "Beginning of initial solution calculations". The equilibration of the system with the given amounts of gypsum and anhydrite at 25 o C is the first batch-reaction step, which is displayed after the heading "Beginning of batch-reaction calculations". Immediately following this heading, the batch-reaction step is identified, followed by a list of the identity of the keyword data used in the calculation. In this example, the solution composition stored as number 1, the pure-phase assemblage stored as number 1, and the reaction temperatures stored as number 1 are used in the calculation. Conceptually, the solution and the pure phases are put together in a beaker, which is regulated to 25 o C, and allowed to react to system equilibrium.
Under the subheading "Phase assemblage", the saturation indices and amounts of each of the phases defined by EQUILIBRIUM_PHASES are listed. In the first batch-reaction step, the final phase assemblage contains no anhydrite, which is undersaturated with respect to the solution (saturation index equals -0.22), and 1.985 mol of gypsum, which is in equilibrium with the solution (saturation index equals 0.0). All of the anhydrite has dissolved and most of the calcium and sulfate have reprecipitated as gypsum. The "Solution composition" indicates that 15.64 mmol/kgw of calcium and sulfate remain in solution, which defines the solubility of gypsum in pure water. However, the total number of moles of each constituent in the aqueous phase is only 15.08 because the mass of water is only 0.9645 kg ("Description of solution"). In precipitating gypsum (CaSO 4 . 2H 2 O), water has been removed from solution. Thus, the mass of solvent water is not constant in batch-reaction calculations; reactions and waters of hydration in dissolving and precipitating phases may increase or decrease the mass of solvent water.
The saturation indices for all of the batch-reaction steps are plotted in figure 5. In each step, pure water was reacted with the phases at a different temperature (the reactions are not cumulative). The default database for PHREEQC indicates that gypsum is the stable phase (saturation index equals 0.0) at temperatures below about 57 o C; above this temperature, anhydrite is calculated to be the stable phase.
Table 14. --Selected output for example 2
-------------------------------------------
Beginning of initial solution calculations.
-------------------------------------------
Initial solution 1. Pure water
-----------------------------Solution composition------------------------------
Elements Molality Moles
Pure water
----------------------------Description of solution----------------------------
pH = 7.000
pe = 4.000
Activity of water = 1.000
Ionic strength = 1.001e-07
Mass of water (kg) = 1.000e+00
Total alkalinity (eq/kg) = 1.082e-10
Total carbon (mol/kg) = 0.000e+00
Total CO2 (mol/kg) = 0.000e+00
Temperature (deg C) = 25.000
Electrical balance (eq) = -1.082e-10
Percent error, 100*(Cat-|An|)/(Cat+|An|) = -0.05
Iterations = 0
Total H = 1.110124e+02
Total O = 5.550622e+01
----------------------------Distribution of species----------------------------
Log Log Log
Species Molality Activity Molality Activity Gamma
OH- 1.002e-07 1.001e-07 -6.999 -6.999 -0.000
H+ 1.001e-07 1.000e-07 -7.000 -7.000 -0.000
H2O 5.551e+01 1.000e+00 0.000 0.000 0.000
H(0) 1.416e-25
H2 7.079e-26 7.079e-26 -25.150 -25.150 0.000
O(0) 0.000e+00
O2 0.000e+00 0.000e+00 -42.080 -42.080 0.000
------------------------------Saturation indices-------------------------------
Phase SI log IAP log KT
H2(g) -22.00 -22.00 0.00 H2
H2O(g) -1.51 0.00 1.51 H2O
O2(g) -39.12 44.00 83.12 O2
-----------------------------------------
Beginning of batch-reaction calculations.
-----------------------------------------
Reaction step 1.
Using solution 1. Pure water
Using pure phase assemblage 1.
Using temperature 1.
-------------------------------Phase assemblage--------------------------------
Moles in assemblage
Phase SI log IAP log KT Initial Final Delta
Anhydrite -0.22 -4.58 -4.36 1.000e+00 -1.000e+00
Gypsum 0.00 -4.58 -4.58 1.000e+00 1.985e+00 9.849e-01
-----------------------------Solution composition------------------------------
Elements Molality Moles
Ca 1.564e-02 1.508e-02
S 1.564e-02 1.508e-02
----------------------------Description of solution----------------------------
pH = 7.067 Charge balance
pe = 10.686 Adjusted to redox equilibrium
Activity of water = 1.000
Ionic strength = 4.178e-02
Mass of water (kg) = 9.645e-01
Total alkalinity (eq/kg) = 1.122e-10
Total carbon (mol/kg) = 0.000e+00
Total CO2 (mol/kg) = 0.000e+00
Temperature (deg C) = 25.000
Electrical balance (eq) = -1.082e-10
Percent error, 100*(Cat-|An|)/(Cat+|An|) = -0.00
Iterations = 19
Total H = 1.070728e+02
Total O = 5.359671e+01
----------------------------Distribution of species----------------------------
Log Log Log
Species Molality Activity Molality Activity Gamma
OH- 1.417e-07 1.167e-07 -6.849 -6.933 -0.084
H+ 9.957e-08 8.575e-08 -7.002 -7.067 -0.065
H2O 5.551e+01 9.996e-01 -0.000 -0.000 0.000
Ca 1.564e-02
Ca+2 1.045e-02 5.176e-03 -1.981 -2.286 -0.305
CaSO4 5.191e-03 5.242e-03 -2.285 -2.281 0.004
CaOH+ 1.204e-08 1.001e-08 -7.919 -7.999 -0.080
CaHSO4+ 3.166e-09 2.633e-09 -8.499 -8.580 -0.080
H(0) 4.383e-39
H2 2.192e-39 2.213e-39 -38.659 -38.655 0.004
O(0) 1.685e-15
O2 8.424e-16 8.505e-16 -15.074 -15.070 0.004
S(-2) 0.000e+00
HS- 0.000e+00 0.000e+00 -117.646 -117.731 -0.084
H2S 0.000e+00 0.000e+00 -117.860 -117.856 0.004
S-2 0.000e+00 0.000e+00 -123.270 -123.582 -0.312
S(6) 1.564e-02
SO4-2 1.045e-02 5.075e-03 -1.981 -2.295 -0.313
CaSO4 5.191e-03 5.242e-03 -2.285 -2.281 0.004
HSO4- 5.088e-08 4.231e-08 -7.293 -7.374 -0.080
CaHSO4+ 3.166e-09 2.633e-09 -8.499 -8.580 -0.080
------------------------------Saturation indices-------------------------------
Phase SI log IAP log KT
Anhydrite -0.22 -4.58 -4.36 CaSO4
Gypsum 0.00 -4.58 -4.58 CaSO4:2H2O
H2(g) -35.51 -35.51 0.00 H2
H2O(g) -1.51 -0.00 1.51 H2O
H2S(g) -116.86 -158.45 -41.59 H2S
O2(g) -12.11 71.01 83.12 O2
Sulfur -87.23 -122.94 -35.71 S