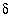 -MnO
2
) and its application to a column experiment, Geochimica et Cosmochimica Acta, v.63, p. 3039-3048.
-MnO
2
) and its application to a column experiment, Geochimica et Cosmochimica Acta, v.63, p. 3039-3048.Aagaard, P. and Helgeson, H.C., 1982. Thermodynamic and kinetic constraints on reaction rates among minerals and aqueous solutions-I. theoretical considerations. American Journal of Science, v. 282, p. 237-285.
Allison, J.D., Brown, D.S., and Novo-Gradac, K.J., 1990, MINTEQA2/PRODEFA2--A geochemical assessment model for environmental systems--version 3.0 user's manual: Environmental Research Laboratory, Office of Research and Development, U.S. Environmental Protection Agency, Athens, Georgia, 106 p.
Appelo, C.A.J., 1994a, Cation and proton exchange, pH variations, and carbonate reactions in a freshening aquifer: Water Resources Research, v. 30, p. 2793-2805.
Appelo, C.A.J., 1994b. Some calculations on multicomponent transport with cation exchange in aquifers: Ground Water, v. 32, p. 968-975.
Appelo, C.A.J., Beekman, H.E. and Oosterbaan, A.W.A., 1984, Hydrochemistry of springs from dolomite reefs in the southern Alps of Northern Italy: International Association of Hydrology, Scientific Publication 150, p. 125-138.
Appelo, C.A.J., and Postma, D., 1999, A consistent model for surface complexation on birnessite (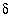 -MnO
2
) and its application to a column experiment, Geochimica et Cosmochimica Acta, v.63, p. 3039-3048.
-MnO
2
) and its application to a column experiment, Geochimica et Cosmochimica Acta, v.63, p. 3039-3048.
Appelo, C.A.J., and Postma, D., 1993, Geochemistry, groundwater and pollution: Rotterdam, A.A. Balkema, 536 p.
Ball, J.W. and Nordstrom, D.K., 1991, WATEQ4F--User's manual with revised thermodynamic data base and test cases for calculating speciation of major, trace and redox elements in natural waters: U.S. Geological Survey Open-File Report 90-129, 185 p.
Barrodale, I., and Roberts, F.D.K., 1980, L1 solution to linear equations subject to linear equality and inequality constraints: Association for Computing Machinery, Transactions on Mathematical Software, v. 6, p. 231-235.
Borkovec, Michal, and Westall, John, 1983, Solution of the \Poisson-Boltzmann equation for surface excesses of ions in the diffuse layer at the oxide-electrolyte interface: Journal of Electroanalytical Chemistry, v. 150, p. 325-337.
Carpenter, A.B., 1978, Origin and chemical evolution of brines in sedimentary basins: Thirteenth Annual Forum on the Geology of Industrial Minerals, eds. Johnson, K.S. and Russell, J.A., Oklahoma Geological Survey Circular 79, p. 60-77.
Cash, J.R. and Karp, A.H., 1990, A Variable Order Runge-Kutta Method for Initial Value Problems with Rapidly Varying Right-Hand Sides: Transactions on Mathematical Software, v. 16, no. 3, p. 201-222.
Charlton, S.R., Macklin, C.L., and Parkhurst, D.L., 1997, PHREEQCI--A graphical user interface for the geochemical computer program PHREEQC: U.S. Geological Survey Water-Resources Investigations Report 97-4222, 9 p.
Crank, J., 1975, The mathematics of diffusion, 2nd ed., Oxford Press, 414 p.
Davis, J.A., and Kent, D.B, 1990, Surface complexation modeling in aqueous geochemistry, in Hochella, M.F., and White, A.F., eds., Mineral-Water Interface Geochemistry: Washington D.C., Mineralogical Society of America, Reviews in Mineralogy, v. 23, Chapt. 5, p.177-260.
Delany, J.M., Puigdomenech, I. and Wolery, T.J., 1986. Precipitation kinetics option for the EQ6 geochemical reaction path code. Lawrence Livermore National Laboratory, University of California, Livermore, 44 p.
De Marsily, G., 1986. Quantitative hydrogeology: Orlando, Academic Press, 440 p.
Dzombak, D.A., and Morel, F.M.M., 1990, Surface complexation modeling--Hydrous ferric oxide: New York, John Wiley, 393 p.
Fehlberg, E., 1969. Klassische Runge-Kutta-Formeln fünfter und siebenter Ordnung mit Schrittweiten-Kontrolle: Computing, v. 4, p. 93-106.
Gaines, G.L., and Thomas, H.C., 1953, Adsorption studies on clay minerals. II. A formulation of the thermodynamics of exchange adsorption: Journal of Chemical Physics, v. 21, p. 714-718.
Garrels, R.M., and Christ, C.L., 1965, Solutions, minerals, and equilibria: New York, Harper and Row, 450 p.
Garrels, R.M, and Mackenzie, F.T., 1967, Origin of the chemical composition of springs and lakes, in Equilibrium concepts in natural water systems: American Chemical Society, Advances in Chemistry Series no. 67, p. 222-242.
Glynn, P.D., 1990, Modeling solid-solution reactions in low-temperature aqueous systems, in Bassett, R.L. and Melchior, D. eds., Chemical modeling in aqueous systems II: Washington D.C., American Chemical Society Symposium Series 416, Chapt. 6, p. 74-86.
Glynn, P.D., 1991, MBSSAS: A code for the computation of Margules parameters and equilibrium relations in binary solid-solution aqueous-solution systems: Computers and Geosciences, v. 17, no. 7, p. 907-966.
Glynn, P.D., and Parkhurst, D.L., 1992, Modeling non-ideal solid-solution aqueous-solution reactions in mass-transfer computer codes, in Kharaka, Y.K., and Maest, A.S. eds., Proceedings of the 7th International Symposium on Water-Rock Interaction: Park City, Utah, July 13-18, 1992, Balkema, Rotterdam, p. 175-179.
Glynn, P.D., and Reardon, E.J., 1990, Solid-solution aqueous-solution equilibria--Thermodynamic theory and representation: American Journal of Science, v. 290, p. 164-201.
Glynn, P.D., Reardon, E.J., Plummer, L.N., and Busenberg, Eurybiades, 1990, Reaction paths and equilibrium end-points in solid solution aqueous-solution systems: Geochimica et Cosmochimica Acta, v. 54, p. 267-282.
Harvie, C.E., Moller, N., and Weare, J.H., 1984, The prediction of mineral solubilities in natural waters: The Na-K-Mg-Ca-H-Cl-SO 4 -OH-HCO 3 -CO 3 -CO 2 -H 2 O system to high ionic strengths at 25 o C: Geochimica et Cosmochimica Acta, v. 48, p. 723-751.
Harvie, C.E., and Weare, J.H., 1980, The prediction of mineral solubilities in natural waters: The Na-K-Mg-Ca-Cl-SO 4 -H 2 O system from zero to high concentration at 25 o C: Geochimica et Cosmochimica Acta, v. 44, p. 981-997.
Helgeson, H.C., Brown, T.H., Nigrini, A., and Jones, T.A., 1970, Calculation of mass transfer in geochemical processes involving aqueous solutions: Geochimica et Cosmochimica Acta, v. 34, p. 569-592.
Helgeson, H.C., Garrels, R.M., and Mackenzie, F.T., 1969, Evaluation of irreversible reactions in geochemical processes involving minerals and aqueous solutions - II. Applications: Geochimica et Cosmochimica Acta, v. 33, p. 455-481.
Langmuir, Donald, 1997, Aqueous environmental geochemistry: Upper Saddle River, New Jersey, Prentice Hall, 600 p.
Lapidus, L., and Amundson, N.R., 1952, Mathematics of adsorption in beds. VI. The effect of longitudinal diffusion in ion-exchange and chromatographic columns: Journal of Physical Chemistry, v. 56, p. 984-988.
Lindstrom, F.T., Haque, R., Freed, V.H. and Boersma, L., 1967, Theory on the movement of some herbicides in soils--Linear diffusion and convection of chemicals: Environmental Science and Technology, v. 1, p. 561-565.
Mosier, E.L., Papp, C.S.E., Motooka, J.M., Kennedy, K.R., and Riddle, G.O., 1991, Sequential extraction analyses of drill core samples, Central Oklahoma Aquifer: U.S. Geological Survey Open-File Report 91-347, 42 p.
Nordstrom, D.K., Plummer, L.N., Langmuir, Donald, Busenberg, Eurybiades, May, H.M., Jones, B.F., and Parkhurst, D.L., 1990, Revised chemical equilibrium data for major water-mineral reactions and their limitations, in Bassett, R.L. and Melchior, D. eds., Chemical modeling in aqueous systems II: Washington D.C., American Chemical Society Symposium Series 416, Chapt. 31, p. 398-413.
Nordstrom, D.K., Plummer, L.N., Wigley, T.M.L., Wolery, T.J., Ball, J.W., Jenne, E.A., Bassett, R.L., Crerar, D.A., Florence, T.M., Fritz, B., Hoffman, M., Holdren, G.R., Jr., Lafon, G.M., Mattigod, S.V., McDuff, R.E., Morel, F., Reddy, M.M., Sposito, G., and Thrailkill, J., 1979, A comparison of computerized chemical models for equilibrium calculations in aqueous systems: in Chemical Modeling in aqueous systems, speciation, sorption, solubility, and kinetics, Jenne, E.A., ed., Series 93, American Chemical Society, p. 857-892.
Parkhurst, D.L., 1995, User's guide to PHREEQC--A computer program for speciation, reaction-path, advective-transport, and inverse geochemical calculations: U.S. Geological Survey Water-Resources Investigations Report 95-4227, 143 p.
Parkhurst, D.L., 1997, Geochemical mole-balance modeling with uncertain data: Water Resources Research, v. 33, no. 8, p. 1957-1970.
Parkhurst, D.L., Christenson, Scott, and Breit, G.N., 1996, Ground-water-quality assessment of the Central Oklahoma Aquifer--Geochemical and geohydrologic investigations: U. S. Geological Survey Water-Supply Paper 2357-C, 101 p.
Parkhurst, D. L., Plummer, L.N., and Thorstenson, D.C., 1982, BALANCE--A computer program for calculating mass transfer for geochemical reactions in ground water: U.S. Geological Survey Water-Resources Investigations Report 82-14, 29 p.
Parkhurst, D.L., Thorstenson, D.C., and Plummer, L.N., 1980, PHREEQE--A computer program for geochemical calculations: U.S. Geological Survey Water-Resources Investigations Report 80-96, 195 p. (Revised and reprinted August, 1990.)
Pitzer, K.S., 1979, Theory--Ion interaction approach: in R.M. Pytkowicz, ed., Activity Coefficients in Electrolyte Solutions, v. 1, CRC Press, Inc., Boca Raton, Florida, p. 157-208.
Plummer, L.N., 1984, Geochemical modeling: A comparison of forward and inverse methods, in Hitchon, B., and Wallick, E.I., eds., First Canadian/American Conference on Hydrogeology, Practical Applications of Ground Water Geochemistry: Worthington, Ohio, National Water Well Association, p. 149-177.
Plummer, L.N. and Back, W.W. 1980, The mass balance approach--Application to interpreting the chemical evolution of hydrologic systems: American Journal of Science, v. 280, p. 130-142.
Plummer, L.N., Busby, J.F., Lee, R.W., and Hanshaw, B.B., 1990, Geochemical modeling of the Madison aquifer in parts of Montana, Wyoming, and South Dakota: Water Resources Research, v. 26, p. 1981-2014.
Plummer, L.N., and Busenberg, Eurybiades, 1987, Thermodynamics of aragonite-strontianite solid solutions--Results from stoichiometric dissolution at 25 and 76 C: Geochimica et Cosmochimica Acta, v. 51, p. 1393-1411.
Plummer, L.N., Parkhurst, D.L., Fleming, G.W., and Dunkle, S.A., 1988, A computer program incorporating Pitzer's equations for calculation of geochemical reactions in brines: U.S. Geological Survey Water-Resources Investigations Report 88-4153, 310 p.
Plummer, L.N., Parkhurst, D.L., and Thorstenson, D.C., 1983, Development of reaction models for groundwater systems: Geochimica et Cosmochimica Acta, v. 47, p. 665-685.
Plummer, L.N., Prestemon, E.C., and Parkhurst, D.L., 1991, An interactive code (NETPATH) for modeling net geochemical reactions along a flow path: U.S. Geological Survey Water-Resources Investigations Report 91-4087, 227 p.
Plummer, L.N., Prestemon, E.C., and Parkhurst, D.L., 1994, An interactive code (NETPATH) for modeling net geochemical reactions along a flow path, version 2.0: U.S. Geological Survey Water-Resources Investigations Report 94-4169, 130 p.
Plummer, L.N., Wigley, T.M.L., and Parkhurst, D.L., 1978, The kinetics of calcite dissolution in CO 2 -water systems at 5 to 60 C and 0.0 to 1.0 atm CO2: American Journal of Science, v. 278, p. 179-216.
Press, W.H., Teukolsky, S.A., Vetterling, W.T., and Flannery, B.P., 1992, Numerical Recipes in C--The Art of Scientific Computing, second edition: Cambridge University Press, 994 p.
Robie, R.A., Hemingway, B.S., and Fisher, J.R., 1978, Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (10 5 pascals) pressure and at higher temperatures: U.S. Geological Survey Bulletin 1452, 456 p.
Russell, E.W., 1973. Soil conditions and plant growth: Longman, London, 849 p.
Schecher, W.D., and McAvoy, D.C., 1991, MINEQL + --A chemical equilibrium program for personal computers--User's manual version 2.1: Edgewater, Maryland, Environmental research Software.
Singer, P.C. and Stumm, W., 1970, Acid mine drainage--the rate limiting step: Science, v. 167, p. 1121-1123.
Sverdrup, H.U., 1990, The kinetics of base cation release due to chemical weathering: Lund University Press, Lund, 246 p.
Tebes-Steven, Caroline, and Valocchi, A.J., 1997, Reactive transport simulation with equilibrium speciation and kinetic biodegradation and adsorption/desorption reactions: A Workshop on Subsurface Reactive Transport Modeling, Pacific Northwest National Laboratory, Richland, Washington, October 29-November 1, 1997 (http://terrassa.pnl.gov:2080/~kash/workshop/bmark.htm).
Tebes-Stevens, C., Valocchi A.J., VanBriesen J.M., Rittmann B.E., 1998, Multicomponent transport with coupled geochemical and microbiological reactions--model description and example simulations: Journal of Hydrology, v. 209, p. 8-26.
Toride, N., Leij, F.J., and Van Genuchten, M.T., 1993, A comprehensive set of analytical solutions for non-equilibrium solute transport with first-order decay and zero-order production: Water Resources Research, v. 29, p. 2167-2182.
Toride, N., Leij, F.J., and Van Genuchten, M.T., 1995, The CXTFIT code for estimating transport parameters from laboratory or field tracer experiments, version 2.0: U.S. Salinity Laboratory Research Report 137, Riverside, California.
Truesdell, A.H., and Jones, B.F., 1974, WATEQ, A computer program for calculating chemical equilibria of natural waters: Journal of Research, U.S. Geological Survey, v. 2, p. 233-274.
Van Cappellen, P. and Wang, Y., 1996, Cycling of iron and manganese in surface sediments: American Journal of Science, v. 296, p. 197-243.
Van Genuchten, M.Th., 1985, A general approach for modeling solute transport in structured soils: IAH Memoirs, v. 17, p. 513-526.
Waite, T.D., Davis, J.A., Payne, T.E., Waychunas, G.A., and Xu, N., 1994, Uranium(VI) adsorption to ferrihydrite--Application of a surface complexation model: Geochimica et Cosmochimica Acta, v. 59, p. 5465-5478.
Williamson, M.A. and Rimstidt, J.D., 1994, The kinetics and electrochemical rate-determining step of aqueous pyrite oxidation: Geochimica et Cosmochimica Acta, v. 58, p. 5443-5454.
Wolery, T. J., 1979, Calculation of chemical equilibrium between aqueous solution and minerals--The EQ3/6 software package: Lawrence Livermore National Laboratory Report UCRL-52658, Livermore, CA.
Wolery, T. J., Jackson, K.J., Bourcier, W.L., Bruton, C.J., Viani, B.E., Knauss, K.G., and Delany, J.N., 1990, Current status of the EQ3/6 software package for geochemical modeling, in Melchior, D.C., and Bassett, R.L., eds., Chemical Modeling of Aqueous Systems II: Washington D. C., American Chemical Society Symposium Series 416, p. 104-116.
Yanenko, N., 1971, The method of fractional steps: Springer, New York.
Yeh, G.T., and Tripathi, V.S., 1989, A critical evaluation of recent developments in hydrogeochemical transport models of reactive multichemical components: Water Resources Research, v. 25, p. 93-108.