Explanation
Keyword for the data block. No other data are input on the keyword line.
Association reaction for aqueous species. The defined species must be the first species to the right of the equal sign. The association reaction must precede any identifiers related to the aqueous species. The association reaction is an identity reaction for each primary master species.
log_k --Identifier for log K at 25 °C. Optionally, -log_k , logk , -l [ og_k ], or -l [ ogk ].
log K --Log K at 25 °C for the reaction. Log K must be 0.0 for primary master species. Default is 0.0.
Line 3: delta_h enthalpy, [ units ]
delta_h --Identifier for enthalpy of reaction at 25 °C. Optionally, -delta_h , deltah , -d [ elta_h ], or -d [ eltah ].
enthalpy --Enthalpy of reaction at 25 °C for the reaction. Default is 0.0 kJ/mol.
units --Default units are kilojoules per mole. Units may be calories, kilocalories, joules, or kilojoules per mole. Only the energy unit is needed (per mole is assumed) and abbreviations of these units are acceptable. Default units are kJ/mol. Explicit definition of units for all enthalpy values is recommended. The enthalpy of reaction is used in the Van’t Hoff equation to determine the temperature dependence of the equilibrium constant. Internally, all enthalpy calculations are performed with the units kJ/mol.
Line 4: -analytic A 1 , A 2 , A 3 , A 4 , A 5 , A 6
-analytic --Identifier for coefficients for an analytical expression for the temperature dependence of log K . Optionally, analytical_expression , a_e , ae , -a [ nalytical_expression ], -a [ _e ], -a [ e ].
A
1
, A
2
, A
3
, A
4
, A
5
, A
6
--Six values defining log
K
as a function of temperature in the expression 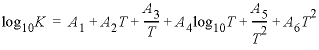 , where
T
is in kelvin.
, where
T
is in kelvin.
Line 5: -gamma Debye-Hückel a, Debye-Hückel b
-gamma
--Indicates activity-coefficient parameters are to be entered. If
-gamma
is entered, then the equation from WATEQ (Truesdell and Jones, 1974) is used, 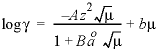 . In this equations,
. In this equations, 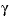 is the activity coefficient,
is the activity coefficient, 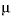 is ionic strength, and
A
and
B
are constants at a given temperature. If
-gamma
is not input for a species, then for a charged species the Davies equation is used to calculate the activity coefficient:
is ionic strength, and
A
and
B
are constants at a given temperature. If
-gamma
is not input for a species, then for a charged species the Davies equation is used to calculate the activity coefficient:
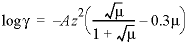 ; for an uncharged species the following equation is used:
; for an uncharged species the following equation is used: 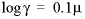 . Optionally,
-g
[
amma
].
. Optionally,
-g
[
amma
].
Debye-Hückel a
--Ion-size parameter
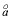 in the WATEQ activity-coefficient equation.
in the WATEQ activity-coefficient equation.
Debye-Hückel b --Parameter b in the WATEQ activity-coefficient equation.
Line 6: -dw diffusion coefficient damp a1 a2
-dw --Identifier for tracer diffusion coefficient. Tracer diffusion coefficients are used in the multicomponent diffusion calculation in TRANSPORT and in calculating the specific conductance of a solution (Basic function SC). Default is 0 m 2 /s (square meter per second) if -dw is not included. Optionally, dw or -dw .
diffusion coefficient --Tracer diffusion coefficient for the species at 25 °C, m 2 /s.
damp --Damping parameter for the temperature effect on viscosity on the diffusion coefficient. Dw(TK) = D * exp(damp / TK - damp / 298.15) * TK * 0.89 / (298.15 * viscos), where Dw is the diffusion coefficient at temperature TK kelvin; D is the diffusion coefficient defined by the first parameter; damp is the damping factor; and viscos is the viscosity of the solution.
a1 --Parameter for the ionic strength effects on the diffusion coefficient of ions in electro-migration. Dw(I) = Dw(TK) * exp(a1 * DH_A * |z| * I^0.5 / (1 + DH_B * I^0.5 * a2 / (1 + I^0.75))), where Dw(I) is the diffusion coefficient corrected for temperature and ionic strength; Dw(TK) is the diffusion coefficient corrected for temperature; a1 is the third parameter defined in -dw; DH_A is the Debye-Huckel A parameter; z is the absolute value of the charge of the aqueous species; I is the ionic strength; DH_B is the Debye-Huckel B parameter; and a2 is the fourth parameter defined in -dw.
a2--Parameter for the ionic strength effects on the diffusion coefficient of ions in electro-migration in the equation above.
Line 7:
-Vm
a1, a2, a3, a4, W,
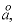 i1, i2, i3, i4
i1, i2, i3, i4
-Vm
--Identifier for parameters used to calculate the specific volume (cm
3
/mol) of aqueous species with a Redlich-type equation (see Redlich and Meyer, 1964). As explained in the following Notes section, the volume of species
i
is calculated, by convention relative to the reference volume of H
+
of 0, as 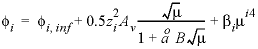 , where the first term of the right-hand side,
, where the first term of the right-hand side, 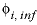 , is the specific volume at infinite dilution, and the second and third terms are functions of the ionic strength
, is the specific volume at infinite dilution, and the second and third terms are functions of the ionic strength  and the ion-size parameter in the extended Debye-Hückel equation,
and the ion-size parameter in the extended Debye-Hückel equation,  and i1, i2, i3 and i4.
and i1, i2, i3 and i4.
The specific volume at infinite dilution is parameterized with SUPCRT92 formulas (Johnson and others, 1992):
where 41.84 transforms cal mol -1 bar -1 (calorie per mole per bar) into cm 3 /mol, P b is pressure in bar, T K is temperature in kelvin, W x Q Born is the Born volume, calculated from W and the pressure dependence of the dielectric constant of water.
The second term contains A v , the Debye-Hückel limiting slope, which is calculated as a function of temperature and pressure, and the extended Debye-Hückel equation (see the Notes).
The coefficient 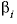 is calculated as
is calculated as 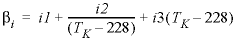 .
.
a1, a2, a3, a4, W,
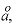 i1, i2, i3, i4
--Numerical values for parameters a1 to a4 (cal mol
-1
bar
-1
, cal/mol (calorie per mole), cal K mol
-1
bar
-1
[calorie kelvin per mole per bar), cal K mol
-1
[calorie kelvin per mol], respectively), the Born coefficient W (cal/mol), the Debye-Hückel
ion-size parameter
i1, i2, i3, i4
--Numerical values for parameters a1 to a4 (cal mol
-1
bar
-1
, cal/mol (calorie per mole), cal K mol
-1
bar
-1
[calorie kelvin per mole per bar), cal K mol
-1
[calorie kelvin per mol], respectively), the Born coefficient W (cal/mol), the Debye-Hückel
ion-size parameter
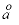 (10-10 m), and i1 (cm
3
/mol), i2 (cm
3
K mol
-1
), i2 (cm
3
K
-1
mol
-1
) and i4 (-), used in the equation for calculating the conventional specific volume of a solute species.
(10-10 m), and i1 (cm
3
/mol), i2 (cm
3
K mol
-1
), i2 (cm
3
K
-1
mol
-1
) and i4 (-), used in the equation for calculating the conventional specific volume of a solute species.
Line 8: -Millero a, b, c, d, e, f
-Millero
--Alternative formulation for calculating the specific volume for the aqueous species (Millero, 2000) by convention relative to the volume of H
+
of 0 at ionic strength of 0. The specific volume for species
i
is calculated according to the formula 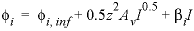 , where
, where 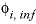 is the specific volume at infinite dilution; A
v
is the Debye-Hückel limiting slope, and
I
is the ionic strength. The volume at infinite dilution is parameterized as
is the specific volume at infinite dilution; A
v
is the Debye-Hückel limiting slope, and
I
is the ionic strength. The volume at infinite dilution is parameterized as 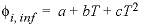 and the coefficient
and the coefficient 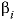 is parameterized as
is parameterized as 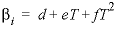 , where
T
is °C. If both
-
Vm and
-
Millero are defined for a species, the numbers from
-
Vm are used. Warning: the applicability of the Millero formulas is limited to T < 50 °C, and the calculated densities may be incorrect at ionic strengths > 1.0 except for NaCl solutions. Optionally,
Millero
or
-Mi
[
llero
].
, where
T
is °C. If both
-
Vm and
-
Millero are defined for a species, the numbers from
-
Vm are used. Warning: the applicability of the Millero formulas is limited to T < 50 °C, and the calculated densities may be incorrect at ionic strengths > 1.0 except for NaCl solutions. Optionally,
Millero
or
-Mi
[
llero
].
a, b, c, d, e, f --Numerical values for parameters a to f in the specific volume equation.
-activity_water --Identifier indicates that the species is an isotopic form of water. The activity coefficient for the species is such that its activity is equal to mole fraction in solution. Optionally, activity_water or -ac [ tivity_water ].
Line 10: -add_logk named log K, coefficient
-add_logk --Identifier defining an additional term for the equilibrium constant of the species. The identifier is used primarily in defining the equilibrium constant for isotopic species that require the addition of an isotopic fractionation factor. Optionally, add_logk , add_log_k , -ad [ d_logk ] or -ad [ d_log_k ].
named log K --Name of an expression defined in a NAMED_EXPRESSIONS data block.
coefficient --Coefficient for the expression named log K ; the value of the expression is multiplied by coefficient and added to the log K for the species.
-llnl_gamma --Identifier for the hard-core diameter in the expression for the activity coefficient in the Lawrence Livermore aqueous model; this identifier can be used only with the Lawrence Livermore National Laboratory aqueous model ( llnl.dat ). Optionally, llnl_gamma or -ll [ nl_gamma ].
diameter --Hard-core diameter for the species.
-co2_llnl_gamma --The activity coefficient for carbon dioxide is used as the activity coefficient for this uncharged species; this identifier can be used only with the Lawrence Livermore National Laboratory aqueous model ( llnl.dat ). Optionally, co2_llnl_gamma or -co [ 2_llnl_gamma ].
-erm_ddl --Identifier for the enrichment factor for a species in the diffuse double layer of surfaces calculated with the -Donnan identifier in the SURFACE data block. Optionally, erm_ddl or -e [ rm_ddl ].
factor --Enrichment factor. Default is 1.0 (unitless).
-no_check --Indicates the reaction equation should not be checked for charge and elemental balance. Generally, equations should be checked for charge and elemental balance. The only exceptions might be polysulfide species that assume equilibrium with a solid phase; this assumption has the effect of removing solid sulfur from the mass-action equation. By default, all equations are checked. However, the identifier -mole_balance is needed to ensure that the proper number of atoms of each element are included in mole-balance equations (see -mole_balance ). Optionally, no_check or -n [ o_check ].
Line 15: -mole_balance formula
-mole_balance --Indicates the stoichiometry of the species will be defined explicitly. Optionally, mole_balance , mass_balance , mb , -m [ ole_balance ], -mass_balance , or -m [ b ].
formula --Chemical formula defining the stoichiometry of the species. Normally, both the stoichiometry and mass-action expression for the species are determined from the chemical equation that defines the species. Rarely, it may be necessary to define the stoichiometry of the species separately from the mass-action equation. The polysulfide species provide an example. These species are usually assumed to be in equilibrium with native sulfur. The activity of a pure solid is 1.0, and thus the term for native sulfur does not appear in the mass-action expression (Line 1g). The S 2 - species contains two atoms of sulfur, but the chemical equation indicates it is formed from species containing a total of one sulfur atom. The -mole_balance identifier is needed to give the correct stoichiometry. Note that unlike all other chemical formulas used in PHREEQC, the valence state of the element can and should be included in the formula of Line 15. The example indicates that the polysulfide species will be summed into the S(-2) mole-balance equation.
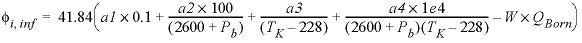 ,
,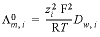 , where
, where 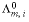 is the molar conductivity (S/m / (mol/m3) equals S m
2
mol
-1
) (siemens square meter per mole), zi is the charge number (unitless) of species i, F is Faraday's constant (C/mol, coulomb per mole), R is the gas constant (J K-1mol-1, joule per kelvin per mole), T is the absolute temperature (K), and Dw, i is the diffusion coefficient (m2/s). PHREEQC calculates the specific conductance of a solution by summing the product of the specific conductivity and the molal concentration of all the species in solution, while correcting the molal concentration with an electrochemical activity coefficient that is derived from a combination of Kohlrausch’s law and the Debye-Hückel equation as explained in http://www.hydrochemistry.eu/exmpls/sc.html (accessed June 25, 2012). The tracer diffusion coefficient is corrected for the temperature
T
(K) of the solution by D
’
w,i
=
(D
w,i
)298
×
is the molar conductivity (S/m / (mol/m3) equals S m
2
mol
-1
) (siemens square meter per mole), zi is the charge number (unitless) of species i, F is Faraday's constant (C/mol, coulomb per mole), R is the gas constant (J K-1mol-1, joule per kelvin per mole), T is the absolute temperature (K), and Dw, i is the diffusion coefficient (m2/s). PHREEQC calculates the specific conductance of a solution by summing the product of the specific conductivity and the molal concentration of all the species in solution, while correcting the molal concentration with an electrochemical activity coefficient that is derived from a combination of Kohlrausch’s law and the Debye-Hückel equation as explained in http://www.hydrochemistry.eu/exmpls/sc.html (accessed June 25, 2012). The tracer diffusion coefficient is corrected for the temperature
T
(K) of the solution by D
’
w,i
=
(D
w,i
)298
× 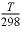 ×
× 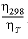 ,
where
η
is the viscosity of water (Atkins and de Paula, 2002).
,
where
η
is the viscosity of water (Atkins and de Paula, 2002).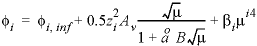 ,
,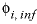 , is the volume at infinite dilution; and the second and third terms are functions of the ionic strength
, is the volume at infinite dilution; and the second and third terms are functions of the ionic strength 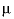 .
.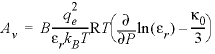 (cm
3
/mol) (mol/kg)
-0.5
,
(cm
3
/mol) (mol/kg)
-0.5
,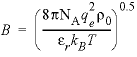 (1/cm)(kg/mol)
0.5
, Avogadro’s number N
A
= 6.022
×
10
23
molecules per mole, the electron charge q
e
= 4.803
×
10
-10
esu (electrostatic unit of charge), the density of pure water
ρ
0
(g/cm
3
), the relative dielectric constant
ε
r
, the Boltzmann constant k
B
= 1.38
×
10
-16
erg/K (erg per kelvin), the temperature T (K), the pressure P (atm), and the compressibility of pure water
κ
0
(atm
-1
). PHREEQC calculates the relative dielectric constant as a function of temperature and pressure, as well as its pressure dependence, according to Bradley and Pitzer (1979), and the density of pure water along the saturation line with equation 2.6 of Wagner and Pruss (2002) and at higher pressures and temperatures with interpolation functions based on IAPWS (International Association for the Properties of Water and Steam) (http://www.nist.gov/srd/upload/NISTIR5078-Tab3.pdf) or with the IF97 (http://www.iapws.org/release.htm) polynomial for region 1 (273 < T < 623 °C, P
sat
< P < 100 MPa, megapascal). The Bradley and Pitzer equations also are used to calculate
(1/cm)(kg/mol)
0.5
, Avogadro’s number N
A
= 6.022
×
10
23
molecules per mole, the electron charge q
e
= 4.803
×
10
-10
esu (electrostatic unit of charge), the density of pure water
ρ
0
(g/cm
3
), the relative dielectric constant
ε
r
, the Boltzmann constant k
B
= 1.38
×
10
-16
erg/K (erg per kelvin), the temperature T (K), the pressure P (atm), and the compressibility of pure water
κ
0
(atm
-1
). PHREEQC calculates the relative dielectric constant as a function of temperature and pressure, as well as its pressure dependence, according to Bradley and Pitzer (1979), and the density of pure water along the saturation line with equation 2.6 of Wagner and Pruss (2002) and at higher pressures and temperatures with interpolation functions based on IAPWS (International Association for the Properties of Water and Steam) (http://www.nist.gov/srd/upload/NISTIR5078-Tab3.pdf) or with the IF97 (http://www.iapws.org/release.htm) polynomial for region 1 (273 < T < 623 °C, P
sat
< P < 100 MPa, megapascal). The Bradley and Pitzer equations also are used to calculate 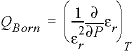 , which is a part of
, which is a part of 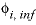 . The specific volumes are used to derive the volume changes of reactions, and hence, the pressure dependency of reaction constants for species, and the pressure dependent solubilities of minerals and gases. The volumes also are used for calculating the density of solutions in PHREEQC as implemented by Vincent Post (Free University, Amsterdam, Netherlands, written commun., 2009) based on the work of Millero (2000). The parameters, entered with the identifier
-Vm
in the
phreeqc.dat and pitzer.dat
databases and commented with “# supcrt modified”, were obtained by least squares fitting of the specific volumes of salts in aqueous solution, compiled by Laliberté (2009), supplemented with data at lower concentrations (omitted by Laliberté (2009)) and at higher temperatures. In the databases, the ion-size parameter
for anions
in the extended Debye-Hückel equation,
. The specific volumes are used to derive the volume changes of reactions, and hence, the pressure dependency of reaction constants for species, and the pressure dependent solubilities of minerals and gases. The volumes also are used for calculating the density of solutions in PHREEQC as implemented by Vincent Post (Free University, Amsterdam, Netherlands, written commun., 2009) based on the work of Millero (2000). The parameters, entered with the identifier
-Vm
in the
phreeqc.dat and pitzer.dat
databases and commented with “# supcrt modified”, were obtained by least squares fitting of the specific volumes of salts in aqueous solution, compiled by Laliberté (2009), supplemented with data at lower concentrations (omitted by Laliberté (2009)) and at higher temperatures. In the databases, the ion-size parameter
for anions
in the extended Debye-Hückel equation,
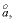 is equal to 0, and for cations equal to the Debye
-Hückel a parameter that is entered with -gamma a. The values defined with
-Millero
in some (now obsolete) databases are, in principle, for the temperature range from 0 to 50
o
C (Millero, 2000) and may be incorrect for high ionic strengths except for solutions containing predominantly alkali cations and chloride anions.
is equal to 0, and for cations equal to the Debye
-Hückel a parameter that is entered with -gamma a. The values defined with
-Millero
in some (now obsolete) databases are, in principle, for the temperature range from 0 to 50
o
C (Millero, 2000) and may be incorrect for high ionic strengths except for solutions containing predominantly alkali cations and chloride anions.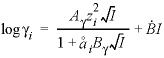 , where
, where 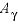 ,
, 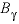 , and
, and 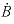 are Debye-Hückel parameters that are functions of temperature as defined in the
are Debye-Hückel parameters that are functions of temperature as defined in the
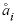 is the hard-core diameter, which is defined for an aqueous species in the
SOLUTION_SPECIES
data block with the
-llnl_gamma
identifier; and
I
is the ionic strength. The activity for an uncharged species in the Lawrence Livermore National Laboratory aqueous model can be set to a function of temperature by using the
-co2_llnl_gamma
identifier. The function of temperature is defined by the
-co2_coefs
identifier in the
is the hard-core diameter, which is defined for an aqueous species in the
SOLUTION_SPECIES
data block with the
-llnl_gamma
identifier; and
I
is the ionic strength. The activity for an uncharged species in the Lawrence Livermore National Laboratory aqueous model can be set to a function of temperature by using the
-co2_llnl_gamma
identifier. The function of temperature is defined by the
-co2_coefs
identifier in the
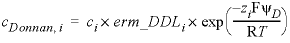 , (4)
, (4)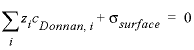 , (5)
, (5)