Keywords
Line 0: MIX 2 Mixing solutions 5, 6, and 7. Line 1a: 5 1.1 Line 1b: 6 0.5 Line 1c: 7 0.3
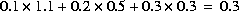 . The moles of all elements are multiplied by the solution's mixing fraction, including hydrogen and oxygen. Thus, the mass of water is effectively multiplied by the same fraction. In the example, if all solutions have 1 kg of water, the total mass of water in the mixture is
. The moles of all elements are multiplied by the solution's mixing fraction, including hydrogen and oxygen. Thus, the mass of water is effectively multiplied by the same fraction. In the example, if all solutions have 1 kg of water, the total mass of water in the mixture is 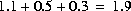 kg and the concentration of sodium would be approximately 0.16 molal. The charge imbalance of each solution is multiplied by the mixing fraction and all the imbalances are then summed to calculate the charge imbalance of the mixture. The temperature of the mixture is approximated by multiplying each solution temperature by its mixing fraction, summing these numbers, and dividing by the sum of the mixing fractions. Other intensive properties of the mixture are calculated in the same way as temperature.
kg and the concentration of sodium would be approximately 0.16 molal. The charge imbalance of each solution is multiplied by the mixing fraction and all the imbalances are then summed to calculate the charge imbalance of the mixture. The temperature of the mixture is approximated by multiplying each solution temperature by its mixing fraction, summing these numbers, and dividing by the sum of the mixing fractions. Other intensive properties of the mixture are calculated in the same way as temperature. This formulation of mixing can be used to approximate constant volume processes if the sum of the mixing fractions is 1.0 and all of the solutions have the same mass of water. The calculations are only approximate in terms of mixing volumes because the summation is actually made in terms of moles (or mass) and the volumes of solutions are not known. Similarly, the formulation for mixing can approximate processes with varying volume, for example, a titration.